Kinetic Theory
AN introduction to Kinetic Theory
Name: Own Teacher
Email: info@ownteacher.com
Created At: 01-11-2023
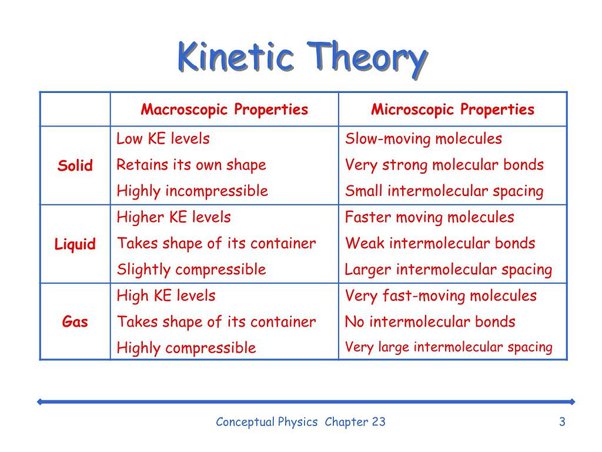
Kinetic Theory is a fundamental model in physics that explains the behavior of gases at the molecular level. It is based on the concept that gases consist of a large number of tiny particles (atoms or molecules) in constant motion. Here is a comprehensive explanation of Kinetic Theory:
1. Molecular Motion:
- Kinetic Theory asserts that gas particles are in constant, random motion. They move in straight lines until they collide with each other or the walls of their container.
2. Ideal Gas Assumption:
- The theory is often applied to ideal gases, which are hypothetical gases that perfectly follow the Kinetic Theory assumptions. Ideal gases have negligible volume and attractive forces between particles.
3. Kinetic Energy:
- Gas particles possess kinetic energy due to their motion. The temperature of a gas is directly related to the average kinetic energy of its particles.
4. Collisions:
- Particles collide with each other and with the walls of their container. These collisions are elastic, meaning no energy is lost.
5. Gas Properties:
- Kinetic Theory helps explain the macroscopic properties of gases, such as pressure, volume, and temperature. It relates pressure to the frequency and force of particle collisions.
6. Gas Laws:
- Kinetic Theory underlies the ideal gas law (PV = nRT) and other gas laws that describe the relationships between pressure, volume, temperature, and the number of moles of a gas.
7. Maxwell-Boltzmann Distribution:
- This statistical distribution describes the distribution of molecular speeds in a gas. It shows that a range of speeds exists among gas particles, with most near the average speed.
8. Deviation from Ideal Behavior:
- Real gases may deviate from ideal behavior due to factors like intermolecular forces and molecular volume. The Van der Waals equation accounts for these deviations.
9. Applications:
- Kinetic Theory is applied in various fields, including chemistry, physics, engineering, and atmospheric science, to understand and predict gas behavior and properties.
10. Experimental Validation:
- Kinetic Theory's predictions have been experimentally validated and are consistent with the observed behavior of gases in real-world situations.
11. Molecular Speed and Temperature:
- The average kinetic energy of gas particles is directly proportional to temperature. As temperature increases, the average speed of gas particles also increases.
12. Effusion and Diffusion:
- Kinetic Theory explains the processes of effusion (gas escaping through a small opening) and diffusion (mixing of gases due to particle motion).
13. Transport Phenomena:
- Kinetic Theory is essential for understanding heat conduction and mass transfer in gases and plays a role in fluid dynamics and plasma physics.
Kinetic Theory provides a fundamental understanding of the behavior of gases and is a cornerstone of thermodynamics and statistical mechanics. It is a powerful tool for modeling and predicting the properties and behavior of gases, particularly in idealized condition.
types of kinetic theory
In the context of physics and thermodynamics, the Kinetic Theory primarily deals with the behavior of gases. While the fundamental principles of Kinetic Theory apply to all gases, there are no distinct "types" of Kinetic Theory. However, there are variations and extensions that consider specific conditions or systems. Some related concepts and variations include:
Classical Kinetic Theory: This is the fundamental theory that describes the behavior of ideal gases, assuming point-like particles with no volume and no intermolecular forces.
Statistical Mechanics: This is a broader framework that includes Kinetic Theory and extends it to describe the behavior of particles in various states of matter, including solids and liquids. It uses statistical methods to understand the behavior of particles at the microscopic level.
Quantum Kinetic Theory: This variation incorporates quantum mechanics into the Kinetic Theory, considering the wave-like nature of particles, their quantized energy levels, and indistinguishability. It's particularly important when dealing with low temperatures and small particles.
Relativistic Kinetic Theory: In high-energy physics and astrophysics, relativistic Kinetic Theory is used to describe the behavior of particles traveling at a significant fraction of the speed of light. It considers the effects of Einstein's theory of relativity.
Polyatomic Gases: Kinetic Theory is often extended to describe the behavior of gases composed of molecules with multiple atoms. These molecules have internal vibrational and rotational degrees of freedom.
Real Gases: Ideal gases, as described by the Kinetic Theory, are an idealization. Real gases deviate from this ideal behavior due to intermolecular forces and the finite volume of gas particles. Modifications to the theory, such as the Van der Waals equation, account for these deviations.
While these variations and extensions are related to the concepts of Kinetic Theory, they are not distinct "types" but rather refinements or adaptations of the theory to address specific conditions or properties of matter. The core principles of Kinetic Theory, which involve the motion of gas particles, remain consistent across these variations.
Cooment List
Leave a Comment.